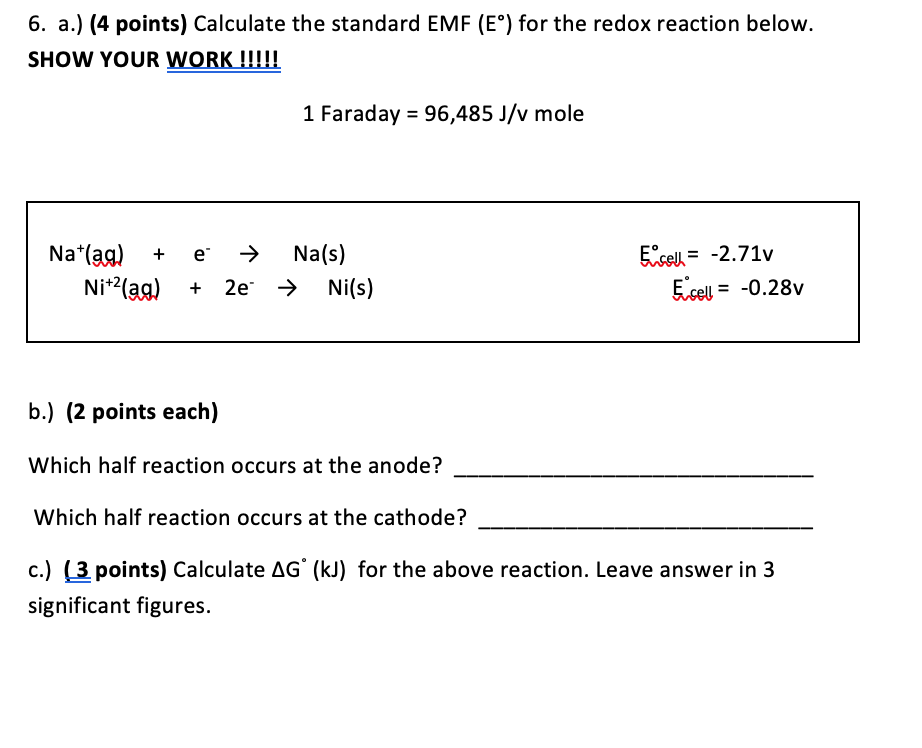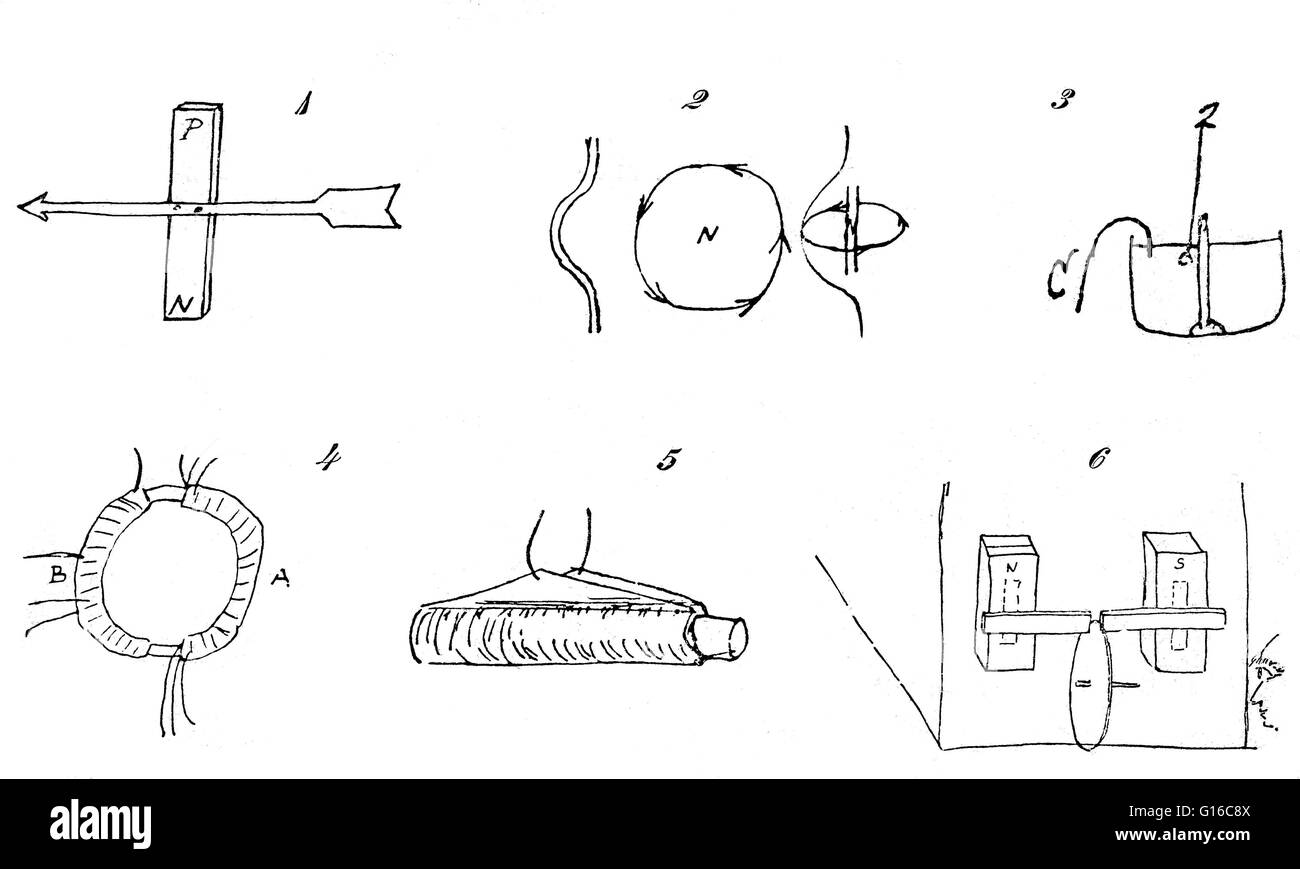
Schizzi da Faraday il diario della mostra la progressione dei suoi esperimenti elettromagnetici. 1) Egli ripete Oersted in seguito alla scoperta del 1820 che un ago magnetico eccepire nei pressi di un

Cyber Nichel Rame 1 Faraday Tessuto EMF Schermatura 50 "x 3' Materiale di blocco del segnale - Plain Weave : Amazon.it: Casa e cucina

SOLVED:The faraday is a unit of charge frequently encountered in electrochemical applications and named for the British physicist and chemist Michael Faraday. It consists of 1 mole of elementary charges. Calculate the
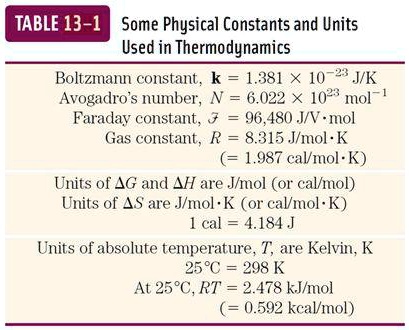
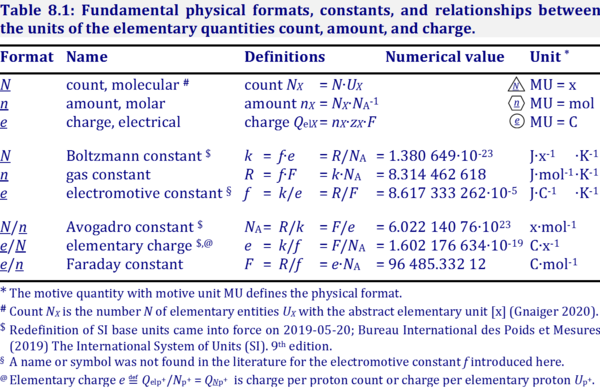
SOLVED: TABLE 13-1: Some Physical Constants and Units Used in Thermodynamics Boltzmann constant, k = 1.381 x 10^(-23) J/K Avogadro's number, N = 6.022 x 10^(23) mol^(-1) Faraday constant, F = 96,480

1 faraday charge is passed through aq solutions of AgNO3, Cuso, and Fecig. The ratio of g equivalents of Ag(s) : Cu(s): Fe(s) deposited is (1)/1: 1:1 (2) 6:3:2 (3) 1:2:3 (4) 1:2:1

1 Suppose we had a 0.3A current producing 15mL of hydrogen gas in 6 min and 30 seconds. How much charge did we have per one mole of electrons? We need. - ppt download

The mass of the substance deposited by 1 faraday of electricity is equal to 11 grams. The value of electrochemical equivalent is: A. 11 B. 11 x 96500 - Correct Answers 11 96500 D. data insufficient