
Which of the following substances have permanent dipole-dipole forces? GeH4; molecular MgCl2; PI3; F2O | Homework.Study.com

Which of the following orders is correct?(1) SbH_{3}> NH_{3}> AsH_{3}> PH_{3} - Boilling point(2) NH_{3}> PH_{3}> AsH_{3}> SbH_{3} - Thermal stability(3) NH_{3}> PH_{3}> AsH_{3}> SbH_{3} - Basic character(4) NH_{3}> PH_{3}> AsH_{3}> SbH_{3} - Bond angle
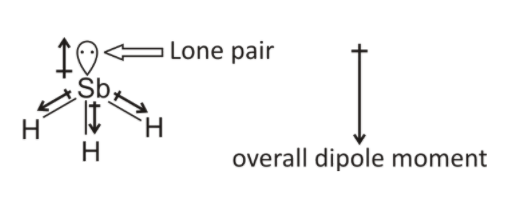
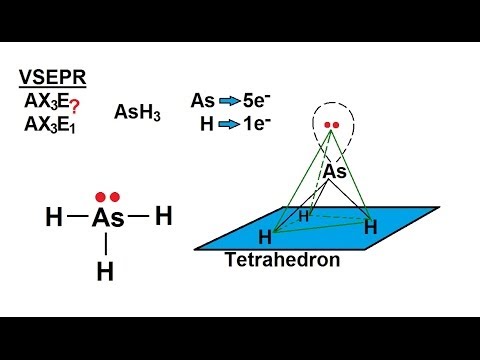
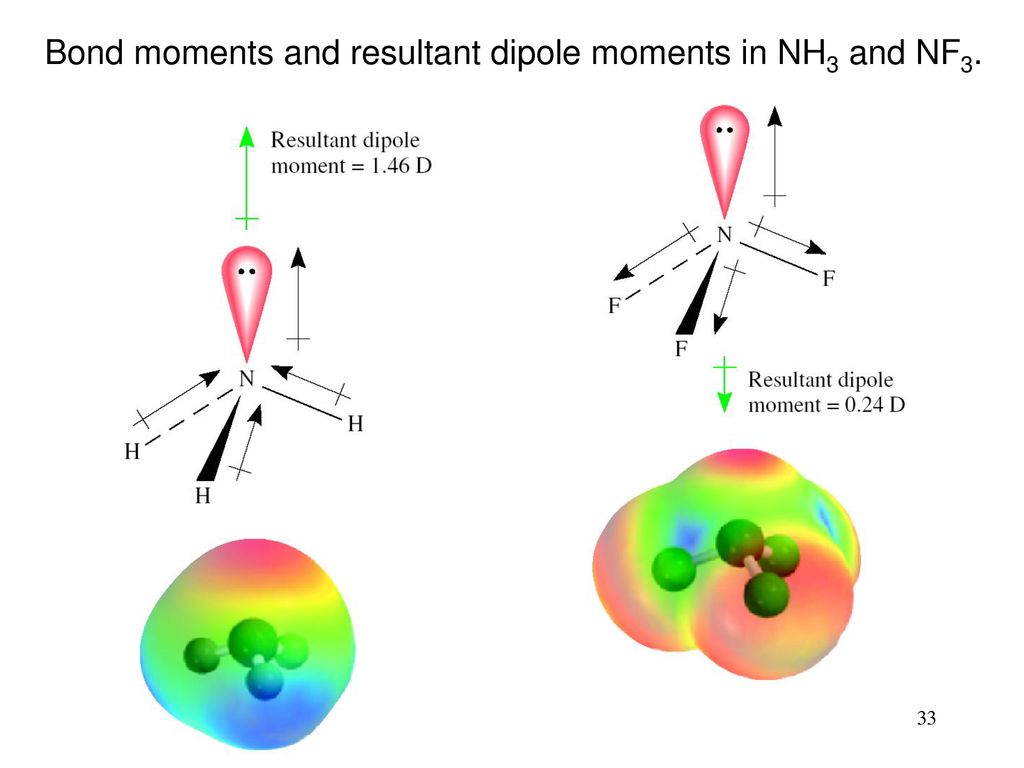
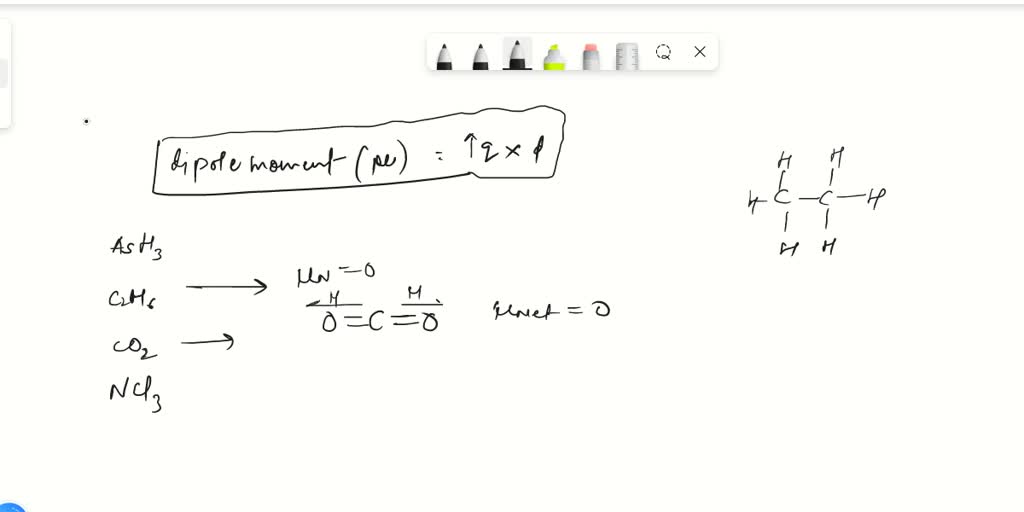
which is the correct order for the following properties?A. Acidity order: $Si{F_4} SiC{l_4} SiB{r_4} Si{I_4}$ B. Melting point: $N{H_3} Sb{H_3} As{H_3} P{H_3}$C. Boiling point: $N{H_3} Sb{H_3} As{H_3} P{H_3}$D. Dipole moment: $N{H_3} Sb{H_3}




![PDF] Absolute local mode vibrational band intensities of AsH3 | Semantic Scholar PDF] Absolute local mode vibrational band intensities of AsH3 | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5609af77a8c2ebdb97a2c432d6643b66067182f6/3-Figure1-1.png)











![PDF] Absolute local mode vibrational band intensities of AsH3 | Semantic Scholar PDF] Absolute local mode vibrational band intensities of AsH3 | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5609af77a8c2ebdb97a2c432d6643b66067182f6/5-Table3-1.png)
