FDA committee meets to debate and vote on Covid booster shots for the general public — 9/17/21 - YouTube

FDA Panel Says Pfizer COVID Booster OK For Older People And Those At High Risk : Coronavirus Updates : NPR

New COVID vaccine: FDA signs off on updated 2023 COVID booster vaccines that target XBB.1.5 Omicron subvariant, EG.5 - ABC7 New York
FDA authorizes Pfizer COVID-19 vaccine boosters for people 65 and older, other at-risk groups - ABC News

CDC recommends new COVID booster for all Americans over 6 months amid rising cases, hospitalizations - ABC News
FDA approves another Covid booster for ages 50 and up; shot would be 4th vaccine dose - syracuse.com
Pfizer asks FDA to authorize Covid booster shots that target omicron BA.5 for people ages 12 and older
FDA vaccine advisers 'disappointed' and 'angry' that early data about new Covid-19 booster shot wasn't presented for review last year | CNN
Tense decision-making as CDC joins FDA in recommending Pfizer booster shot for 65 & up, people at high risk and those with occupational exposure to COVID-19
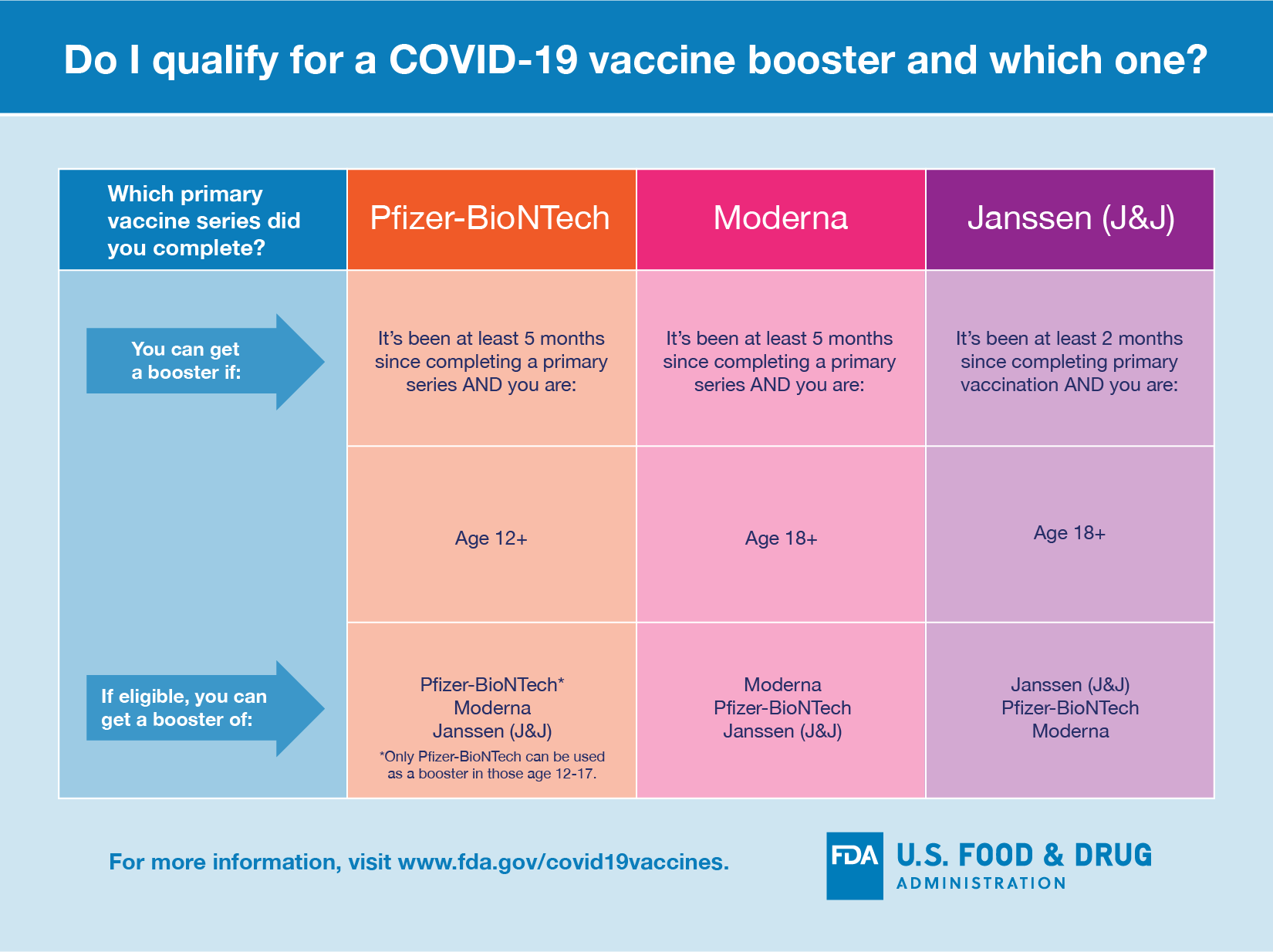
Coronavirus (COVID-19) Update: FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months | FDA
FDA greenlights Pfizer booster shot for certain groups; CDC advisory panel votes to recommend boosters | AHA News