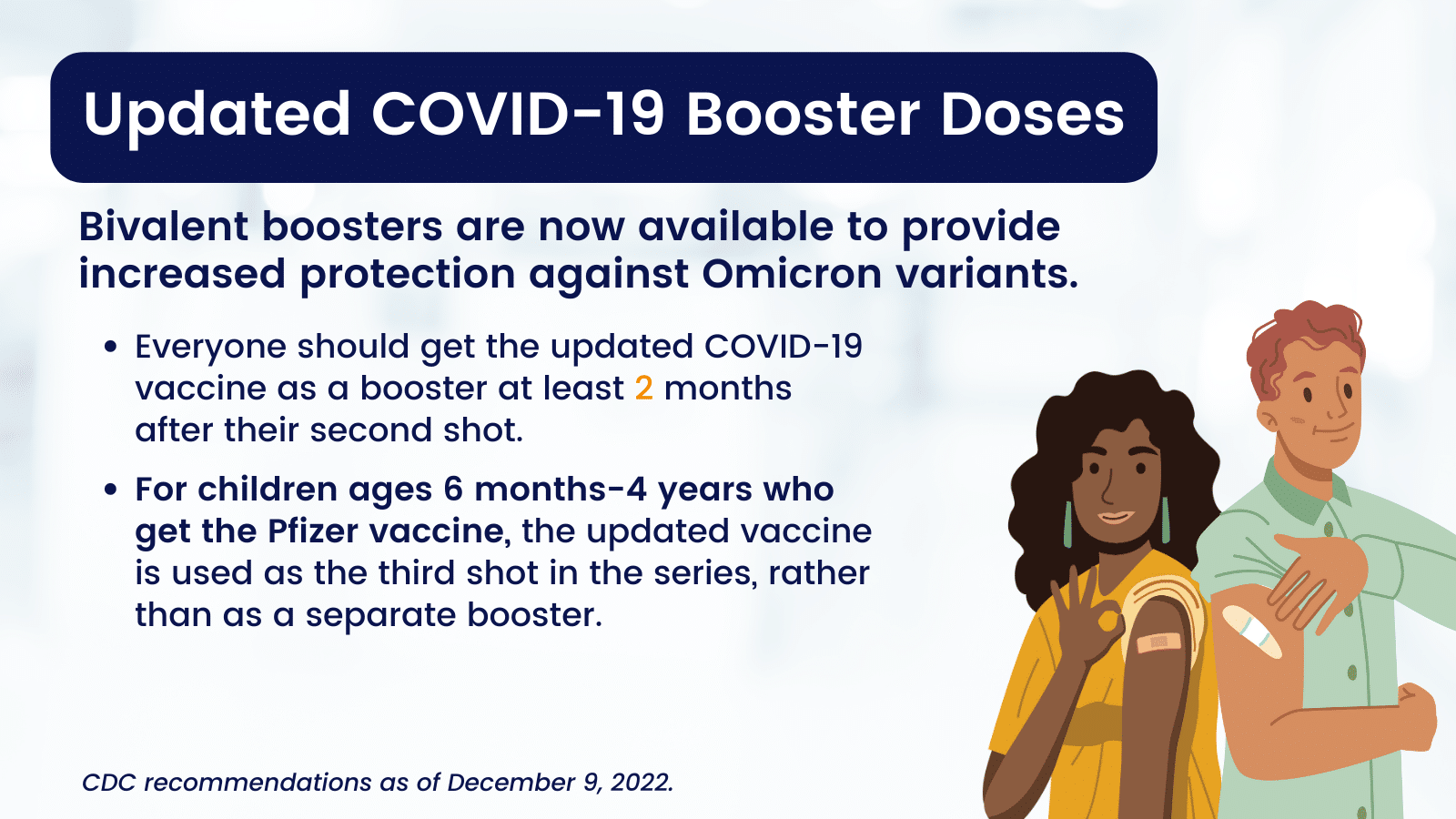
Updated Toolkit: COVID-19 Booster Dose Messaging and Outreach Tools - Public Health Communications Collaborative (PHCC)

If your provider is waiting for Moderna Omicron booster doses, there's plenty of Pfizer – AZ Dept. of Health Services Director's Blog

New Moderna and Pfizer-BioNTech Bivalent COVID-19 Boosters Available at Meharry | Meharry Medical Group
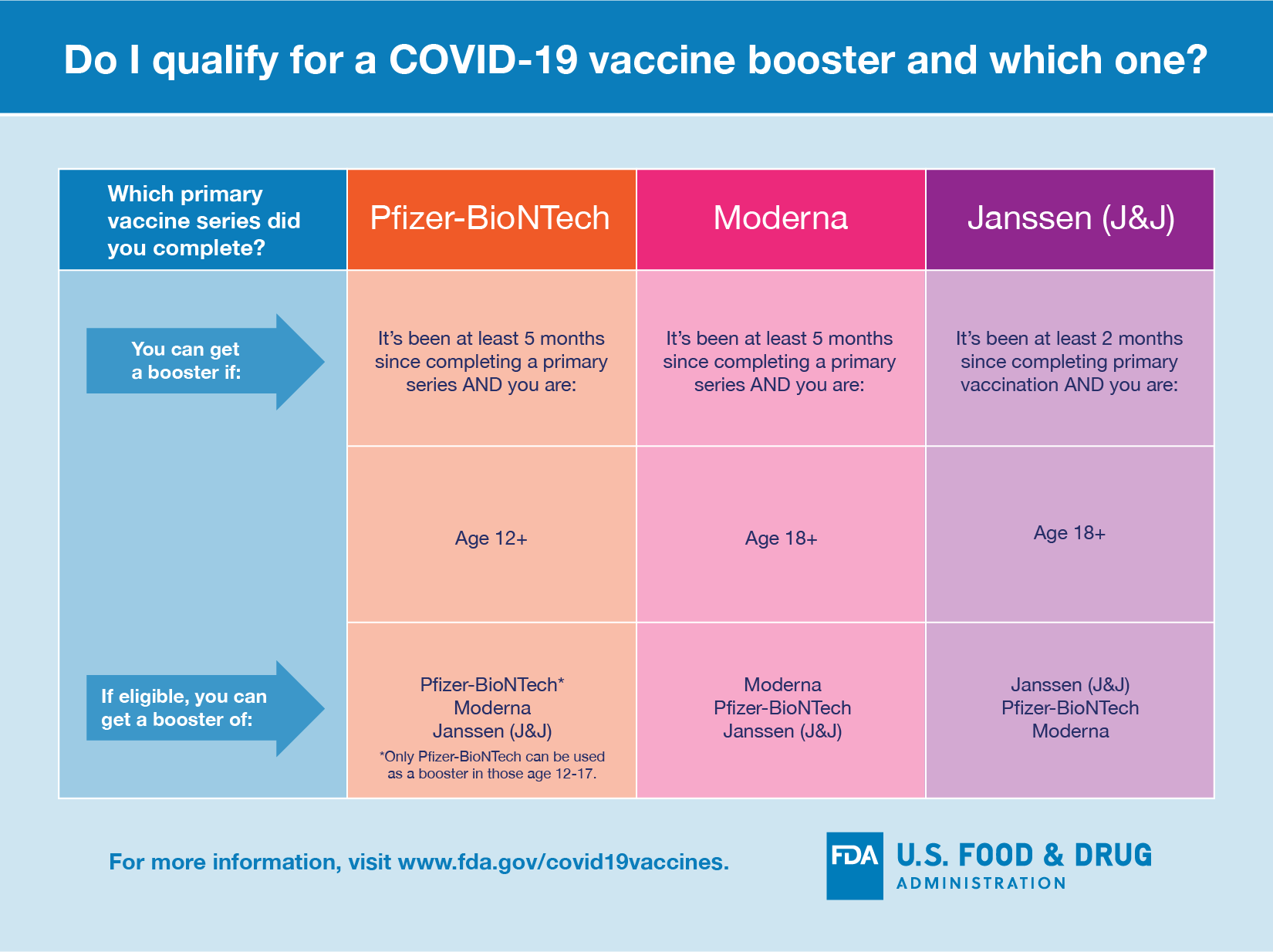
Coronavirus (COVID-19) Update: FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months | FDA